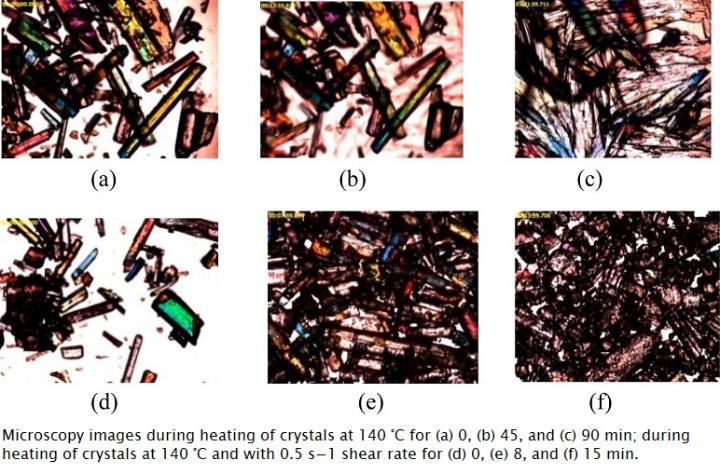
Process induced polymorphic transitions
Researchers at CPES have extensive knowledge of investigating polymorphism. Using a range of specialist analytical techniques the team characterise polymorphs of drug substance and identify transitions between metastable and thermodynamically stable forms.
Pharmaceutical processing has the potential to disrupt the crystal lattice of APIs and has thus gained considerable attention. Different molecular arrangements can provide diverse physical and chemical properties in pharmaceutical substances, thus altering solubility, density, stability, and bioavailability. Several unit operations employed to prepare solid dosage forms, such as grinding, milling, drying, and compression may induce solid-state polymorphic transformation.
The CPES research team has explored various processes including solvent free, scalable thermo-mechanical technology for continuous crystalline transformation using a hot melt extruder.