Micro-Injection Moulded Microneedles for Drug Delivery
The Centre for Polymer Micro and Nanotechnology at the University of Bradford has developed a novel process using micro injection moulding to produce microneedles using a medical grade polymer.
Microneedles are novel minimally invasive drug delivery devices that have the potential to deliver biological molecules such as insulin and vaccines through the skin in a painless manner through self-administration compared to painful hypodermic needle injections administered by a healthcare professional.
Because the length of the microneedle is sufficiently short to avoid stimulating the nerve endings only minimal discomfort is experienced by the patient. In addition, the use of microneedles for the delivery of drugs potentially offers numerous advantages over conventional hypodermic injections for a broad range of agents for short, medium and long term treatments.
By using an array of microneedles designed to penetrate the stratum corneum of the skin, the drug can either act locally or is absorbed by the capillary system (depending on the micro-needles design and the drug characteristics) and is subsequently distributed via the blood circulatory system.
2012 global market figures evaluated the emergent microneedle market at just under $400 million with a promising trajectory as microneedles are targeted not only to the $25 billion global vaccine market, but also to the $120 billion global biologics market.
There are a number of manufacturing solutions to produce microneedles such as wet and dry etching or the machining of metals such as silicon oxide and titanium. However, these methods are not cost effective, clinically effective or easily sterilised for mass production.
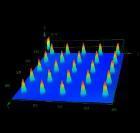
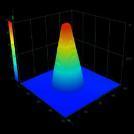
Opportunity
The Centre for Polymer Micro and Nanotechnology at the University of Bradford has developed a novel process using micro injection moulding to produce microneedles using a medical grade polymer. Our solution allows the production of microneedle patches at a low cost while preventing any unwanted effects caused by the possible breakage of the microneedle tips into the skin, thus allowing the drugs to be absorbed through the skin at a controlled delivery rate. This opens up potential opportunities for development of technologies such as theragnostic autonomous delivery devices for example for insulin delivery, where the microneedle could automatically monitor blood glucose levels and intermittently inject the requisite amount of insulin at appropriate times.
IP Status
Relevant background landscape searches have been performed and are indicative that there is freedom to operate in this area using this technology.

