Raman Mapping of Product Formulations
Researchers at the University of Bradford have been at the forefront of innovation in applications of Raman Spectroscopy. Our commercial team has the opportunity to work closely with leading academics to develop new or current methodologies to help resolve your challenges.
Raman Spectroscopy provides a “chemical fingerprint” of a pharmaceutical compound making it a powerful analytical tool for determining molecular composition and structure, identifying solid form (polymorphs, solvates, amorphous form) and assessing other physico-chemical properties that are important for understanding, predicting and controlling drug product performance.
Pharmaceutical Raman Imaging
The Centre has extensive knowledge and experience of using Raman Microscopy to evaluate the physico-chemical properties of pharmaceutical materials. Using 2-dimensional Raman mapping techniques the Centre have previously studied:
- Differences in physical form in pharmaceutical formulations; crystalline vs. amorphous form, polymorph identity, solvated species, co-crystal forms etc.;
- The distribution of one or more active ingredients in a pharmaceutical product, and even distribution of different forms of materials in formulations;
- The PSD of one or more active ingredients in a pharmaceutical product.
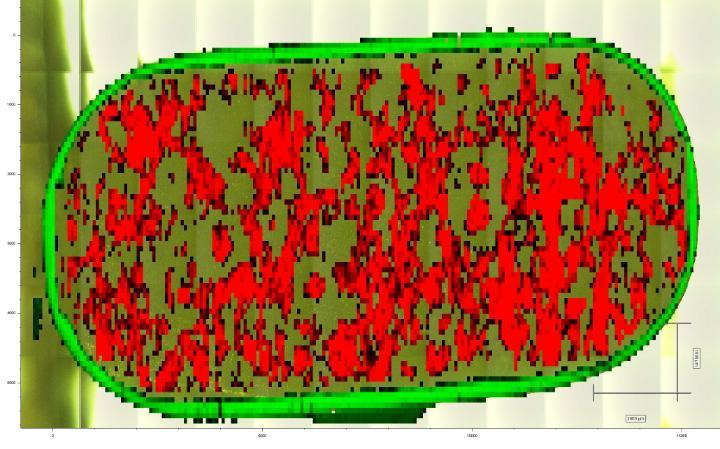
Raman map of a Ibuprofen caplet
